ON THE ISSUE OF HYGIENIC SAFETY OF EMULSION TYPE SAUCES ENRICHED WITH SELENIUM
Abstract
The need to create the sources of bioactive selenium that is suitable for insertion into the daily diet is proved. The fundamental technological scheme of «Selenium» emulsion-type sauce (ETS) production is developed. The ETS is enriched with selenium by using the «Sivoselen Plus» and «Neoselen» selenium-protein dietary supplements (SPDS) as the source of organic selenium. The data confirming essentially complete normalization of the intestinal barrier permeability in experimental rodents are given. The influence of ETS «Selenium» with SPDS «Sivoselen Plus» and «Neoselen» on the state of the intestinal barrier in rats is studied. This is confirmed by the following data: the serum alanine aminotransferase activity in experimental animals compared to the indicators of intact rats of the control group varied slightly during the study period; there were no statistically reliable oscillations of aspartate aminotransferase activity; the alkaline phosphatase activity of serum of rats was not statistically significant differences from those in the control group of rats. The biochemical parameters of white linear rats blood serum are in the range of normalized indicators for healthy animals. The hygienic safety of ETS «Selenium» with SPDS «Sivoselen Plus» and «Neoselen» is studied and proved.
Introduction. Today there are no doubts in the fact that food substantially influences health state of a person. Physiologically good nutrition, from its integral component in the form of high-quality and quantitative providing an organism with necessary nutrients, is capable to support the high level of immunological resistance and antioxidant protection. The essential role in antioxidant cell protection, maintenance of cellular immunity, normal functioning of thyroid, prostatic glands and active course of spermatogenesis is played by selenium [1, page 21]. Shortage aggravates diseases of respiratory organs [2, page 749]. Deficiency of selenium is noted for patients with malignant blood diseases [3, page 4260]. Its experimental deficiency leads to intestines cancer development for rats [4, page 1298] and skin cancer for mice [5, page 2813].
Thus, there is a need for creating sources of biologically active selenium suitable for introduction into daily diet.
Main Part. One of ways to complement the essential micro-nutrients lacking in a human body is regular inclusion in food rations of all the population categories of the specialized foodstuff enriched with vital components. In this regard there is a need of products creation, for example, of the emulsion type sauces (ETS) which will allow to carry out alimentary correction of diseases and pathological states.
The selenium-lack situation in Ukraine tends to deterioration due to reduction of selenium amount in soils and, as a result, in products of plant growing and animal husbandry. Therefore a question of economically developed and at the same time safe food products enriched with organic compounds of selenium becomes timely.
We developed technologies of receiving additives dietary selenium - proteinaceous (SPDS) «Sivoselen Plus» and «Neoselen» [6]. Creation of new SPDS provides interaction of selenium ions source with proteins of whey. Including them in the population diet will allow to support the antioxidant resistance of human body to aggressive environment.
For today the complex of SPDS quality indicators, parameters of their acute toxicity is defined [7, page 222-231]. Toxicity class of additives is 5th, that shows their relative non toxicity.
Also, we developed the production technology of ETS «Selenic» (fig.) enriched with selenium.
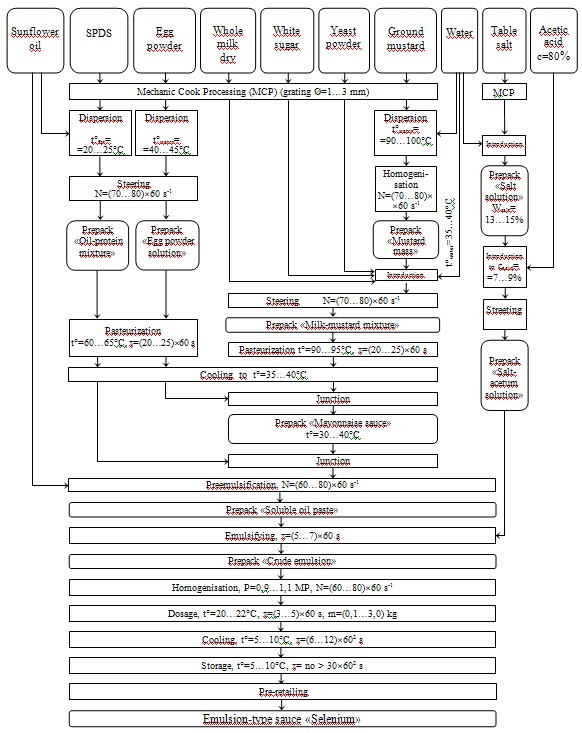
Fig. Process flow diagram of the production of emulsion-type sauce «Selenic»
Принципиальная технологическая схема производства соуса эмульсионного типа «Селеновый»
Apparent from the schematic diagram of ETS production is that the above SPDS are entered into its compounding. The basis of development was formed by the production technology of high-fat mayonnaise «Molochny».
As SPDS «Sivoselen Plus» and «Neoselen» contain enough serumal proteins of milk (mainly laktoalbumina, lactoglobulins) and carbohydrates, it was decided to replace completely powdered skim milk with above-mentioned additives for the purpose of reduction in the final product cost.
At this stage of scientific activity a number of researches proving «appeal» and safety of ETS «Selenic» to the consumer is carried out [8, page 137-141]. But, nevertheless, keen interest causes a question of hygienic safety of this product, its toxi-kinetics and toxicology.
It is known that aminotransferases are key enzymes of a proteinaceous exchange and a link between it and a carbohydrate exchange. The increase in the contents serumal alaninaminotransferaza (ALAT) and an aspartataminotransferaza (ASAT) serves one of the markers of hepatocytes destruction at which there is a strengthened exit of these intracellular enzymes in blood.
Therefore the high level of these enzymes shows cytolytic syndrome. ALAT is more specific marker of liver diseases than ASAT. The Alkaline Phosphatase (AP) is present practically at all tissues of an organism. However blood serum research SP usually is of interest in connection with diagnostics the gepatobiliary diseases. The increase in activity of serumal SP can show violation of biliary tract bile outflow [9, page 455].
Thus, discovering activity of the specified enzymes in blood serum in a complex gains bigger value during assessment of gastrointestinal tract functioning violations and confirmation of structural changes in liver.
Work purpose. Studying of hygienic safety of the emulsion type sauces enriched with selenium became the purpose of this work.
Materials and research methods. Research of safety indicators of ETS enriched with selenium was carried out on samples of white linear rats blood on the basis of the Research center of bio-safety and environmental control of agrarian and industrial complex resources of the Dnepropetrovsk State Agrarian and Economic University.
Experiments were made on 60 white non purebred mature rats divided into the following groups: the first – control (intact animals, n=20), others – animals who were fed with ETS from SPDS «Sivoselen Plus» (n=20) and «Neoselen» (n=20). Before introduction of ETS animals were kept on a hungry diet within 6-8 hours [10, page 5]. Sauces were entered into an animal stomach by means of a gastric probe. Blood-sample was taken in 2-3 h after introduction of ETS and brought to laboratory in 20 min. after.
Defining activity of enzymes in rats blood was carried out on the automatic biochemical Cobas Integra 400 plus analyzer (Hoffmann-La Roche Ltd., Germany) by means of kinetic UF and photometric methods.
The obtained figures statistically processed by means of the descriptive and comparative analysis with use of Student criterion. Distinctions considered reliable if p < 0,05.
Research results and discussion. Results of the biochemical analysis of white linear rats blood serum are given in tab. 1 and 2.
Table 1
Results of complex biochemical research of rats blood indicators (ETS with SPDS «Sivoselen Plus»)
Результаты комплексного биохимического исследования показателей крови крыс (СЭТ с ДДСБ «Сивоселен Плюс»)
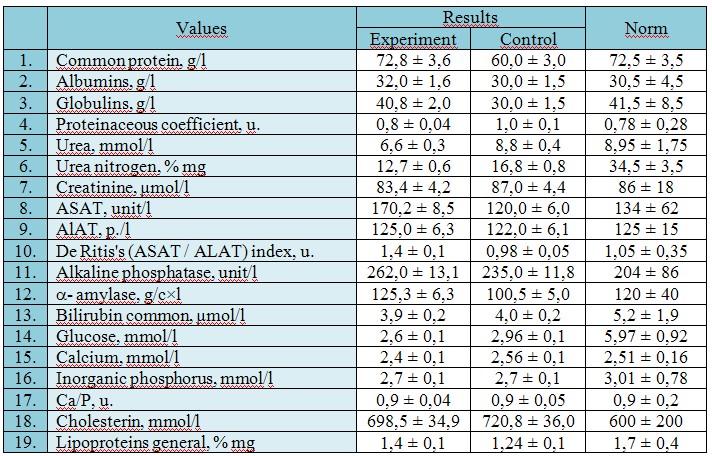
Table 2
Results of complex biochemical research of rats blood indicators (ETS with SPDS «Neoselen»)
Результаты комплексного биохимического исследования показателей крови крыс (СЭТ с ДДСБ «Неоселен»)
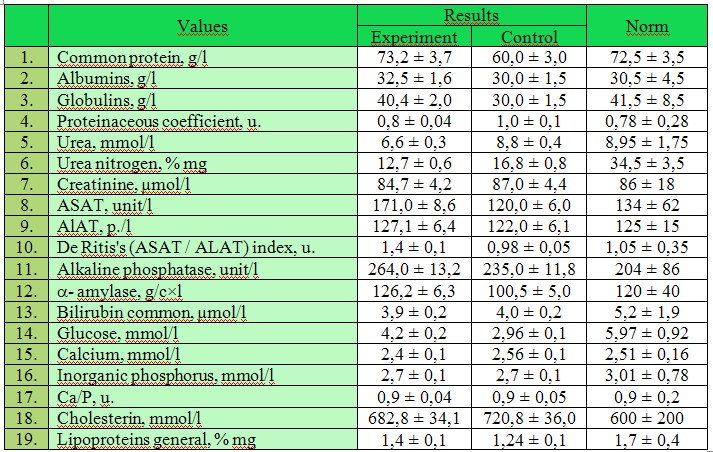
Researching changes of serumal enzymes activity in blood we observed the following picture. Activity the ALAT blood serum of control group intact rats, experimental in comparison with indexes, during research changed slightly. The index varied within 125 ± 4,0 units/l.
Activity of serum ASAT at intact rats of control group and activity of this enzyme in rats to whom ETS with SPDS was injected, in all points of comparison statistically important did not differ. That is, during the whole time of experiment statistically reliable fluctuations of the ASAT activity was not noted.
After introduction of ETS with SPDS activity of SP blood serum in rats it had statistically no significant differences from the corresponding index in rats of control group.
Conclusion. Research of ETS «Selenic» with SPDS «Sivoselen Plus» and «Neoselen» influence on rats intestinal barrier condition showed the almost complete normalization of intestinal barrier transmittance.
Generally, results of biochemical blood serum research of white linear rats are in limits of the normalized indicators for healthy animals. Therefore, ETS with SPDS are sanitary safe foodstuff and can be recommended for special health nutrition.
















Reference lists